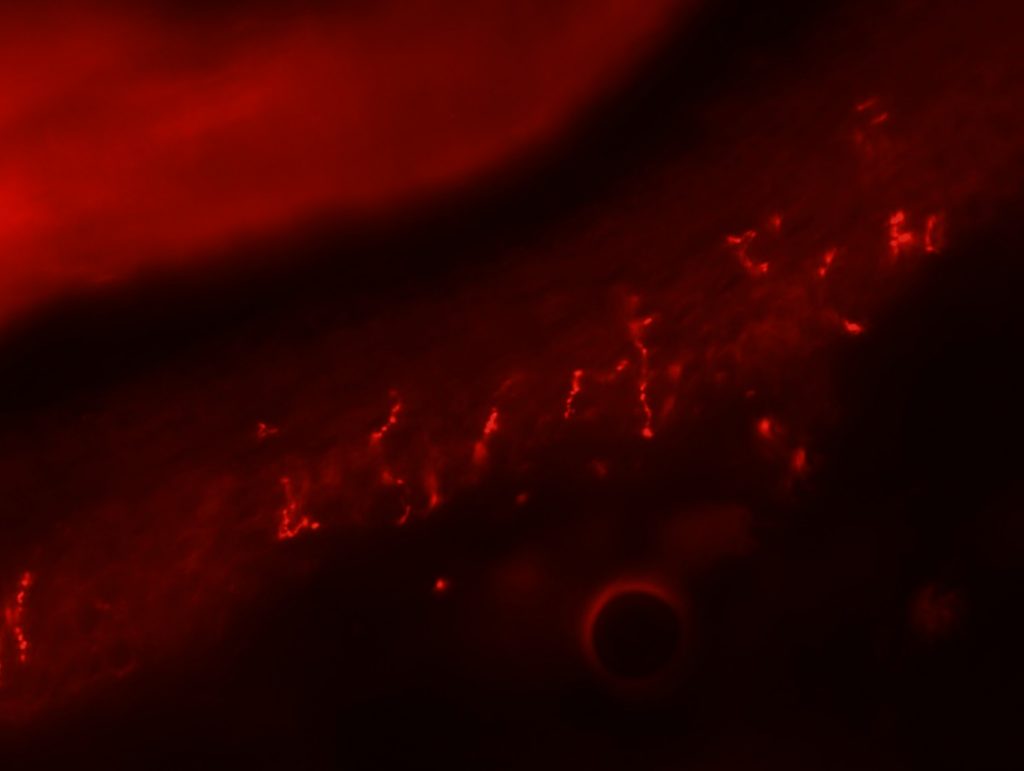
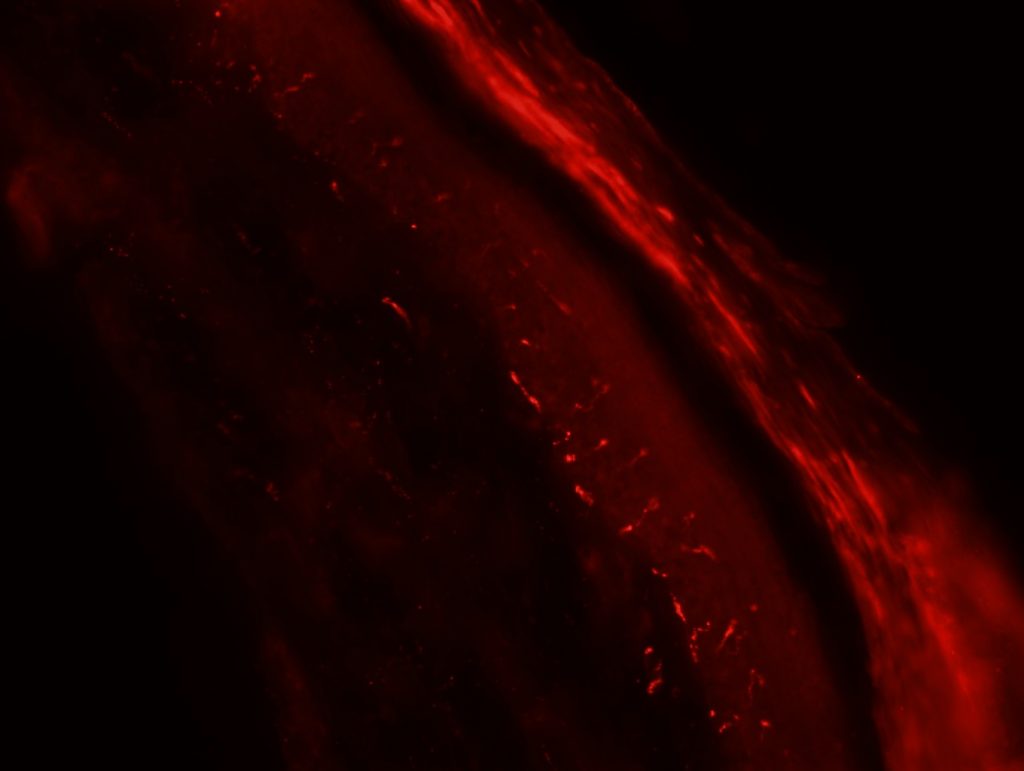
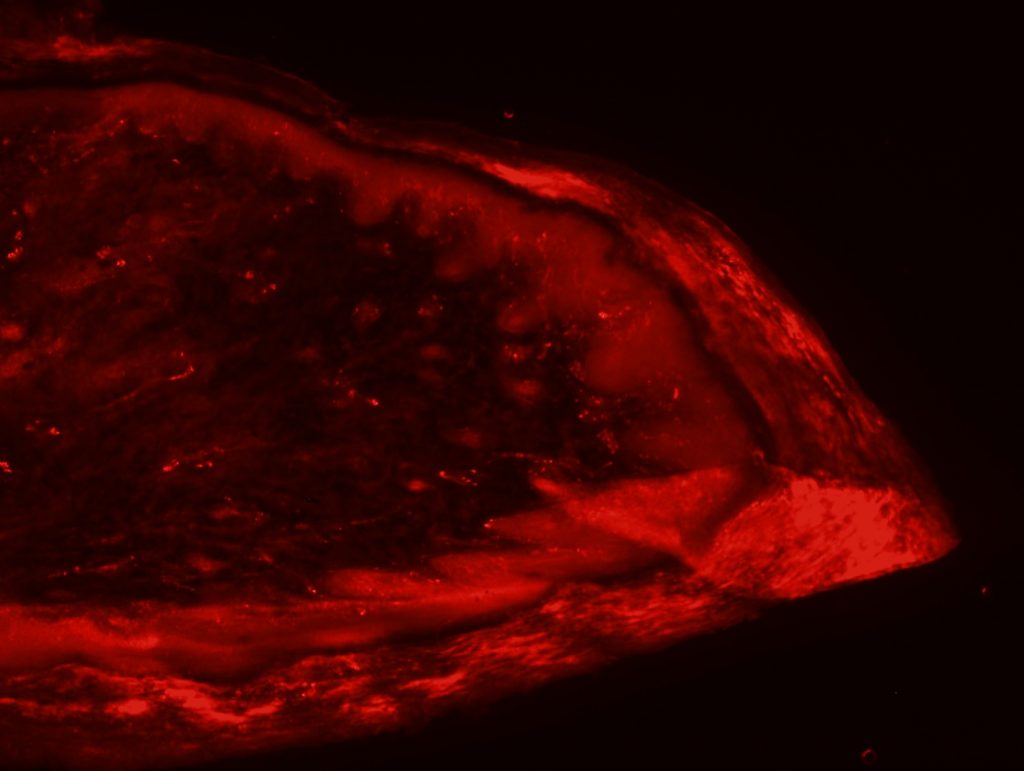
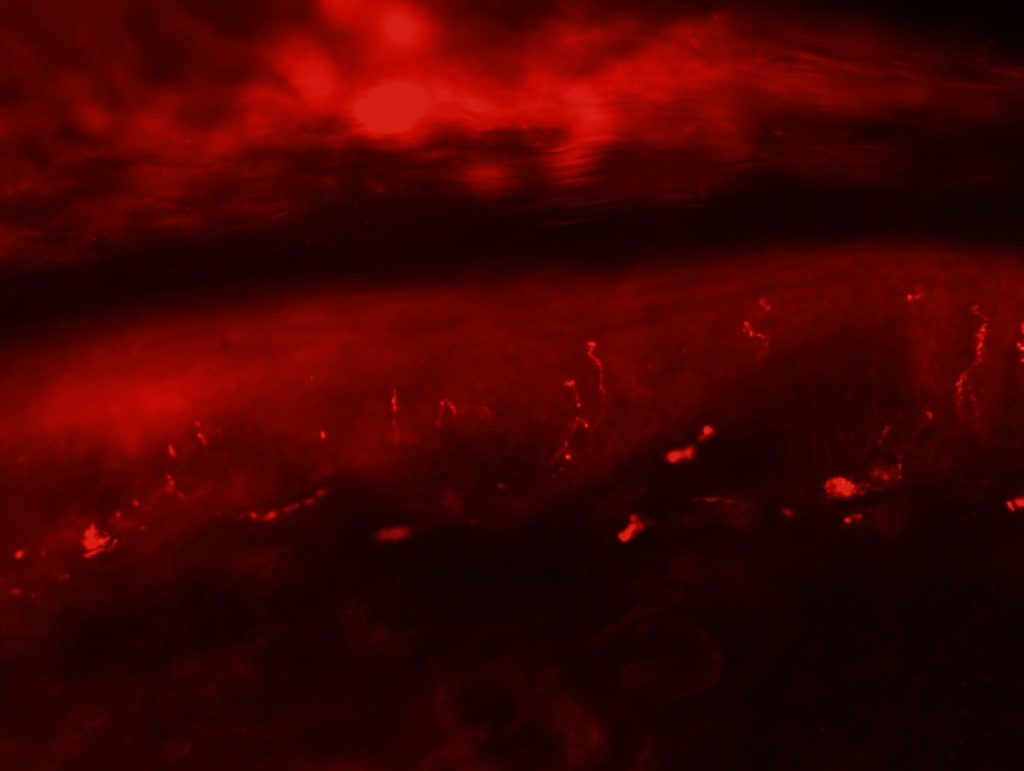
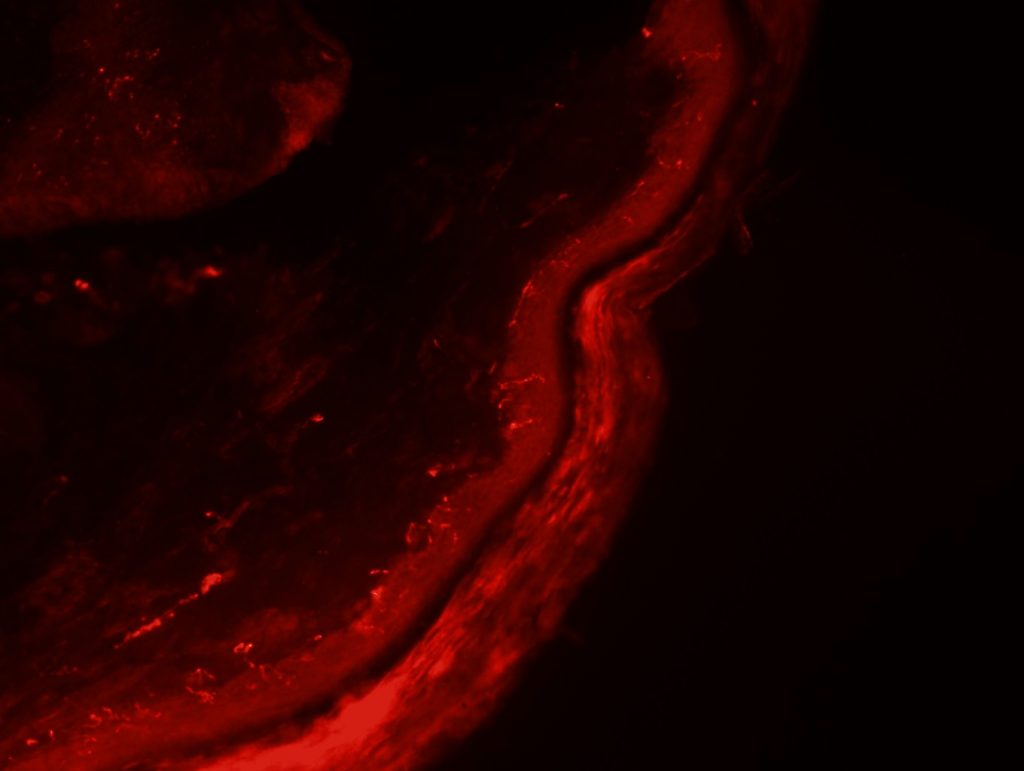
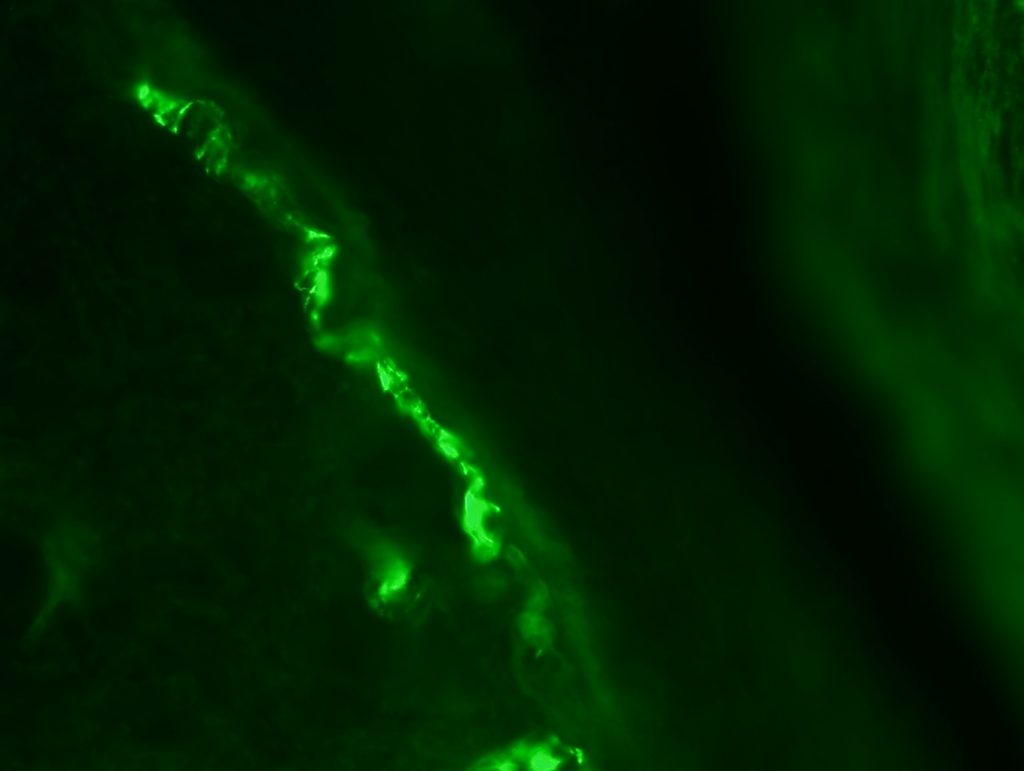
OBJECTIVE: To test skin biopsy procedure in foot pads from a rat. We will test 2 conditions for fixation: 4% PFA in PBS and 4% PFA in phospate buffer. Also, we will test two different autoantibodies: PGP 9.5 for IENFD and MBP for myelinated fibers.
PREPARATION:
- Fix the skin 30 min with 4% PFA in phosphate buffer (in eppendorf)
- Wash the skin in phosphate buffer (x 3, in eppendorf, without agitation)
- Incubate the skin with 10% sucrose at 4ºC overnight
- Embed in Tissue Tek – Freeze in nitrogen with 2-Methylbutan
- Cut the skin at 40um in ultra-frozen slides
- Let the preparation dry for 30 minutes in the air (or more)
- Freeze at -20ºC overnight
IMMUNOFLUORESCENCE (in humid chamber):
1st day:
- Air-dry slides for 30 min and thaw one or two blocking aliquots.
- Outline the sample boundaries with DAKO-PEN.
- Block 30min at room temperature with 10% BSA in PBS.
- Preparation of the primary antibody
a. 1%BSA (1ml 10%BSA+9ml PBS)
b. 1%BSA + 0.3%TritonX (1ml 1%BSA + 3ul TritonX) (vortex).
c. PGP 9.5 (polyclonal rabbit-to-human) (1:200) diluted in 1%BSA + 0.3% TritonX. MBP (polyclonal mouse-to-human) (1:200) diluted in 1%BSA + 0.3%TritonX. - Remove the blocking solution (tapping on paper, no washing).
- Put the primary antibody on the sections (approx. 40uL in each).
- Incubate at room temperature overnight.
2nd day:
- Wash the slides in PBS (x3) for 5 min.
- Preparation of Cy3 conjugate IgG secondary antibody – Dilute Cy3 (Anti-rabbit IgG conj.) in 1%BSA PBS 1:50 for PGP staining and GAM 488 (650318) at different dilutions: 1:100, 1:200 and 1:400.
- Put the secondary antibody on the slide (40uL) for 2h at room temperature and in the dark.
- Wash with PBS x3.
- Cover the slides with Mounting media: Vectashield-DAPI.
- Cover the edges with nail polish or Vitro-Cloud (to prevent the preparations from drying out).
- Store at 4ºC in the dark until reading. Once analyzed, they are frozen at -20ºC, where they can last for years and can be re-analyzed in the future if necessary.
RESULTS: Both fixation methods works well, maybe 4% PFA in phosphate buffer is better than in PBS. PGP 9.5 staining looks well for IENFD counting. I have some doubts on MBP staining (in green). I will perform different stainings to see if it works properly.