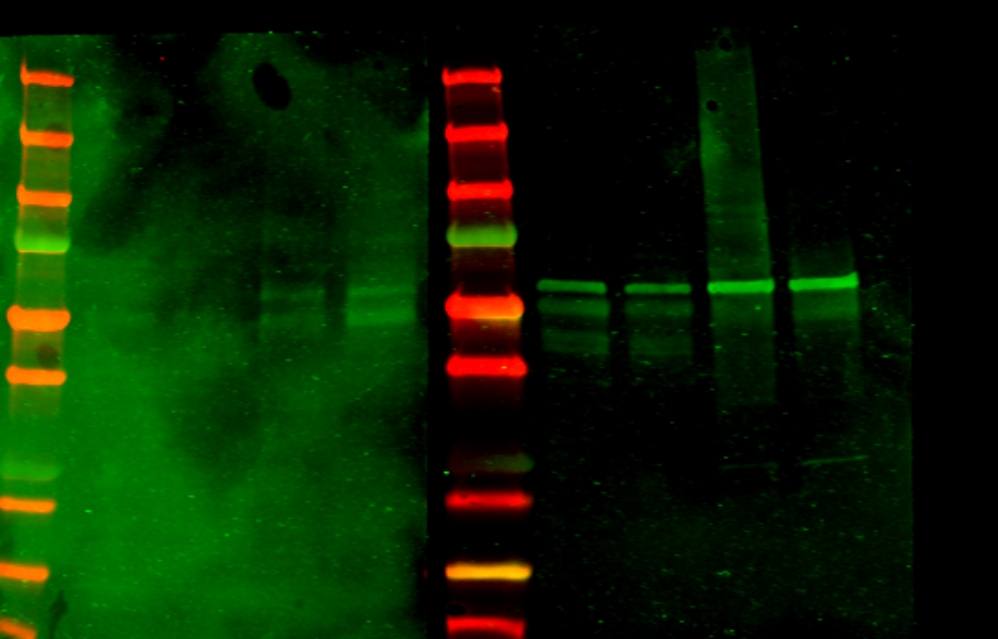
Wells: 30 ug protein/well.
- Marker
- Rat brain
- Rat cerebellum
- non-transfected HEK cells
- FLOT1/2-transfected HEK cells
- Marker
- Rat brain
- Rat cerebellum
- non-transfected HEK cells
- FLOT1/2-transfected HEK cells
Protocol:
- We have used a precast gel (4-15% achrylamide) with 10 wells (Bio-Rad)
- Warm the samples at 96ºC for 5 minutes
- Load samples in the wells (30ul per well)
- Run with running buffer 1x (commercial –> BioRad) at 20 – 40mA
- Transfer to a nitrocellulose membrane with Transblot Turbo (using the commercial buffer) –> mixed proteins protocol
- Blotting: wash the membrane with water and cut it (in this case we have 2 parts)
- Blocking: blocking buffer Casein – PBS 1:1 1h
- Primary antibodies (diluted in Casein-PBS 1:1) –> 1 hour at RT
- Mb1 (from well 1 to 5) –> serum MS 158 1/50
- Mb2 (from well 6 to 10) –> serum negative control 202-02 1/50
- Wash with PBS-tween0’1% (3×5′)
- Secondary antibody (1)
- Mb1 and 2: GAH800 1/7500 (diluted in Casein-PBS tween0’1%): 1h at RT
- Wash with PBS-tween0’1% 1x (2×5′)
- Wash with PBS 1x (1×5′)
- Readwith Odyssey equipment
RESULT
La membrana incubada con el suero 158 se ve muy sucia y no se pueden diferenciar las bandas. De todos modos parece que aparezcan las mismas bandas que en la membrana incubada con el control negativo.
Al correr el tejido o las células, el complejo FLOT1/2 se deshace y puede que los sueros con anticuerpos contra el complejo no se puedan pegar –> Hacer IP y después WB