Objective: To detect CNTN1 antibodies by a lateral flow kit.
Conjugate the capture and the detection antibodies using the lightning-Link® Ulfa-Tag kit and Gold Conjugation Kit (40 nm, 20 OD) respectively, following the instructions below. Allow all of the reagents to warm to room temperature.
1.1 Lightning-Link® Ulfa-Tag Conjugation – protein CNTN1. [CNTN1]=250mg/ml – dilución a 1mg/ml (2uL en 500uL). We use 200uL
- Before you add antibody to the Ulfa-Tag mix, add 1 μL of LL- Modifier reagent for each 10 μL of antibody to be labeled (20uL). Mix gently.
- Remove the screw cap from the vial of Ulfa-Tag mix and pipette the antibody sample (with added LL-Modifier) directly onto the lyophilized material. Resuspend gently by withdrawing and re-dispensing the liquid once or twice using a pipette.
- Place the cap back on the vial and leave the vial standing for 3 hours at room temperature (20-25⁰C). Alternatively, and sometimes more conveniently, conjugations can be set up and left at room temperature overnight, as the longer incubation time has no negative effect on the
conjugate. - After incubating for 3 hours (or more), add 1 μL of LLQuencher reagent for every 10 μL of antibody used (20uL). The conjugate can be used after 30 minutes. No separation steps are necessary.
1.2 Gold Conjugation – RAG488 (A11078) – 2mg/ml
- Dilute your stock detection antibody with the Gold antibody diluent provided in the kit to 0.1 mg/mL recommended concentrations. (1uL in 20uL)
- In a microfuge tube add the Gold reaction buffer and your now diluted antibody according to the table below and mix gently: Gold reaction buffer – 42uL and Diluted antibody -12uL. You will have more mixture than you will actually use in the conjugation reaction. Note: If you wish to examine the effect of varying the amount of antibody, make additional stocks but do not change the volume of antibody added (see table below). In order to vary the amount of antibody added, you must change the concentration of the stock antibody and use a fixed volume.
- Add 45 μL of reaction buffer/antibody mixture to a Mini vial, and reconstitute the Gold by gently pipetting up and down. Leave the reaction for 15 minutes at room temperature.
- After 15 minutes, add 5 μL Gold quencher to stop the reaction, and mix well, but gently.
- Leave the reaction for 5 minutes. You now have 20 OD conjugate (50 μL). Dilute further as required for your application.
Optional (not performed in this assay): - For a conjugate 100% free from unbound antibody we recommend to wash the particles by adding 10 times the volume of the quencher diluted 1:10 in water to the conjugate (i.e. 1 mL 1:10 diluted quencher to 100 μL of conjugate) and then centrifuge it in a microfuge at 9,000 x g for 10 minutes.
- Carefully remove the supernatant, gently tap the pellet and add the quencher diluted 1:10 in water for long term storage in the fridge (up to 1 year) or 1:10 diluted quencher with addition of 0.5 – 2% BSA for LFA or your preferred buffer. It is important to avoid substances that have a very high affinity for Gold (e.g. thiols).
1.2 Dilute the 10x Universal Running Buffer 1:10 with distilled water and add a blocking agent (0.1% BSA final concentration) to obtain 1x Universal Running Buffer + BSA.
1.3 Dilute the Ulfa-Tag conjugated capture antibody to 40 to 150 μg/mL in 1x Universal Running Buffer + BSA.
- Different concentrations: A) 50ug/ml, B)100ug/ml, C) 150ug/ml
1.4 Dilute the Gold-detection antibody to 6 OD in 1x Universal Running Buffer + BSA.
1.5 Dilute the 40 nm Gold-Biotin 10 OD to 1 OD in 1x Universal Running Buffer + BSA (1:10 dilution).
1.6 Dilute your analyte in 1x Universal Running Buffer + BSA.
1.7 We recommend testing each sample in duplicate or triplicate.
For a single strip, prepare the following mix:
5 μL diluted capture Ulfa-Tag-conjugated antibody
5 μL 6 OD 40 nm Gold-detection antibody
5 μL 1 OD 40 nm Gold-Biotin
75 μL analyte solution
- Analytes:
- 1) AF109 0.1mg/ml diluted in universal running buffer + BSA (11.25uL in 213.75uL)
- B)egative control (112.5uL serum and 112.5uL buffer)
1.8 Incubate for 5 minutes.
1.9 In a 96 well plate, load 80 μL of the mix and insert one
Universal LFA strip in each well. Handle the strip from the
wicking pad (thicker pad, made of cellulose), avoid touching
the nitrocellulose, and make sure the sample pad (thinner and
longer pad, made of glass fiber) is dipped into the well.
1.10 Run the mix for 20 minutes.
1.11 Compare the T-line color intensity with the score reported on
the scoring card. If the C-line is not visible the test is not valid
and should be repeated.
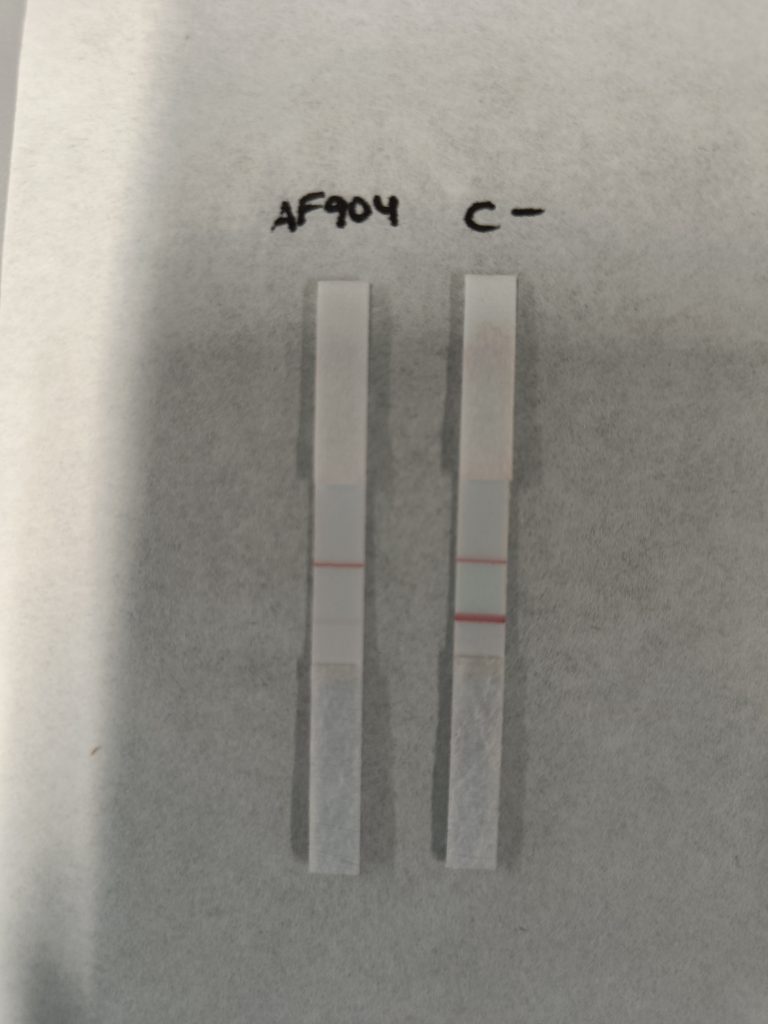
RESULTS: The reaction containing AF904 is negative while the negative control has a positive reaction with a clear T-line. The C-line (control) is positive in both samples. We will contact the cientific support and will repeat the experiment with different conditions, adding the optional steps of washing.